What type of function is Gibbs free energy?
Índice
- What type of function is Gibbs free energy?
- Is Gibbs free energy path independent?
- Is Gibbs energy a state function?
- Is Gibbs free energy a law?
- Why is Gibbs energy useful?
- Is change in free energy a state function?
- Why Gibbs energy is called free energy?
- Is the change in Gibbs free energy dependent on the path?
- Why is Gibbs energy considered a state function?
- What is the relation between the Gibbs potential and temperature?
- What is the formula for Gibbs free energy?
What type of function is Gibbs free energy?
The Gibbs free energy is one of the most important thermodynamic functions for the characterization of a system. It is a factor in determining outcomes such as the voltage of an electrochemical cell, and the equilibrium constant for a reversible reaction.
Is Gibbs free energy path independent?
The ΔG of a reaction is independent of the path (or molecular mechanism) of the transformation. The mechanism of a reaction has no effect on ΔG. For example, the ΔG for the oxidation of glucose to CO2 and H2O is the same whether it occurs by combustion in vitro or by a series of enzyme-catalyzed steps in a cell.
Is Gibbs energy a state function?
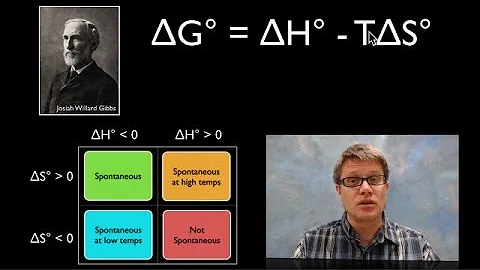
Gibbs Energy is a state function defined as G=H–TS. The practical utility of the Gibbs function is that ΔG for any process is negative if it leads to an increase in the entropy of the world. Thus spontaneous change at a given temperature and pressure can only occur when it would lead to a decrease in G.
Is Gibbs free energy a law?
Derivation of Gibbs Free Energy According to the First Law of Thermodynamics, positive energy flow is when work is done on the system, or heat is added to it, and the total energy change of the universe is zero (the energy gained by the system is lost by the surroundings, and vice versa).
Why is Gibbs energy useful?
The importance of the Gibbs function can hardly be over-stated: it determines whether a given chemical change is thermodynamically possible. Thus, if the free energy of the reactants is greater than that of the products, the entropy of the world will increase and the reaction takes place spontaneously.
Is change in free energy a state function?
Since free energy usually contains potential energy, it is not absolute but depends on the choice of a zero point. Therefore, only relative free energy values, or changes in free energy, are physically meaningful. The free energy is a thermodynamic state function, like the internal energy, enthalpy, and entropy.
Why Gibbs energy is called free energy?
Free energy, called Gibbs free energy (G), is usable energy or energy that is available to do work. Change in gibbs free energy gives the direction and extent of the reaction. Free energy machines do not work. ... It is named after Josiah Willard Gibbs and Hermann von Helmholtz.
Is the change in Gibbs free energy dependent on the path?
- Gibbs free energy is a state function hence it doesn’t depend on the path. So change in Gibbs free energy is equal to the change in enthalpy minus the product of temperature and entropy change of the system.
Why is Gibbs energy considered a state function?
- Gibbs energy is a state function because it is a combination of other state functions, G = H - TS. When you consider a change of state, all you are doing is subtracting the value of G in one state from the value of G in another. So the “delta G” is not a state function, any more the change of temperature, delta T, is a state function.
What is the relation between the Gibbs potential and temperature?
- The Gibbs energy (also known as the Gibbs function or Gibbs Potential) is defined as G = H– TS in which S refers to the entropy of the system. Since H, T and S are all state functions, so is G. Thus for any change in state (under constant temperature), we can write the extremely important relation
What is the formula for Gibbs free energy?
- The Gibbs' free energy is a thermodynamic function of the natural variables, temperature T and pressure P. At constant T and P, though, in a thermodynamically-closed system, the following formulas are useful: dG = dH −T dS − ΔT =0 assumed SdT (1) (recall ΔG = ΔH − T ΔS from general chemistry.)